Trusted By






Quality Care, Trusted Solutions
A biotech facility is a specialized laboratory or manufacturing space where biological research and production activities are conducted.
Quality control (QC) in a biotech facility is essential to ensure that products meet predefined standards of quality, safety, and efficacy.
A nanotech facility is a specialized laboratory or production space dedicated to the research development, and manufacturing.
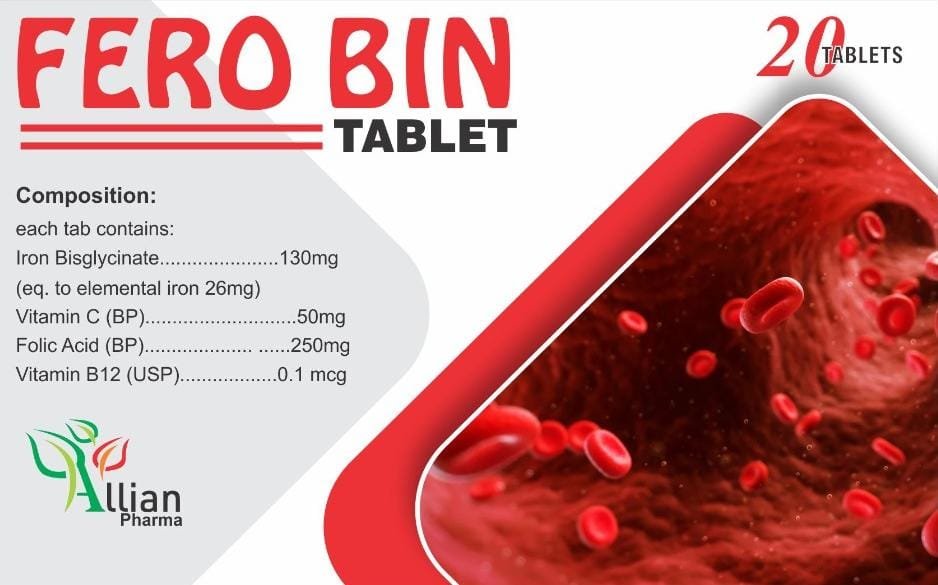
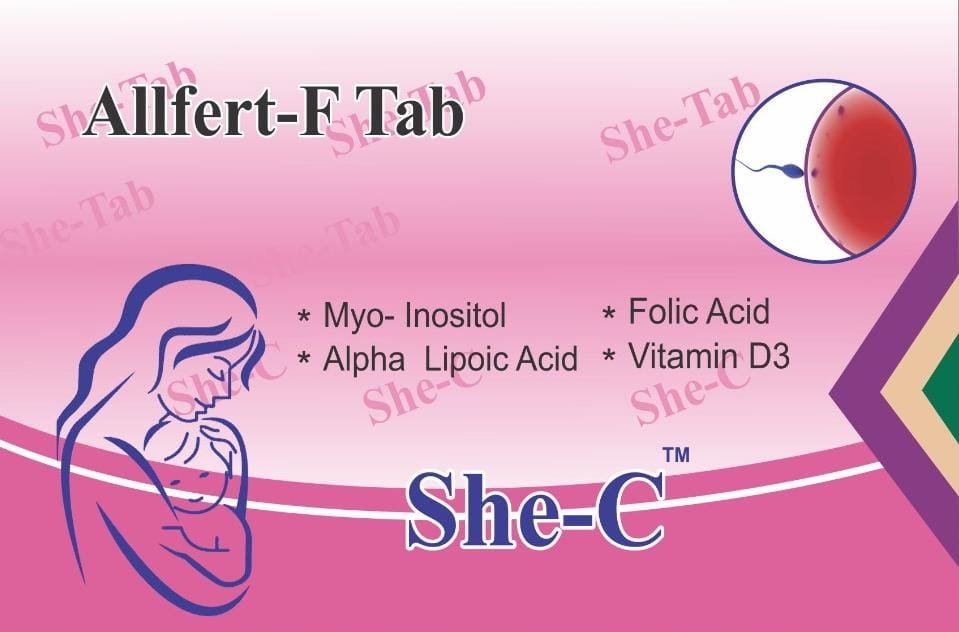
Allian Pharma is a pharmaceutical company specializing in the development, manufacturing, and marketing of innovative pharmaceutical products. The company’s focus spans various therapeutic areas, leveraging advanced research and development (R&D) processes to discover and bring new drugs to market. Their R&D efforts include high-throughput screening for drug discovery, in vitro and in vivo studies for preclinical research, and comprehensive clinical trials to ensure the safety and efficacy of their products. Allian Pharma also emphasizes regulatory compliance, quality control, and state-of-the-art manufacturing practices to produce high-quality pharmaceuticals that meet global standards. Their commitment to innovation and patient care drives their mission to improve health outcomes worldwide.
High-throughput screening and computational chemistry to identify and design new drug candidates
Conducting in vitro and in vivo studies to evaluate the efficacy, safety, and pharmacokinetics of drug candidates
Implementing Phase I, II, and III clinical trials to test drug safety, efficacy, dosage, and side effects in humans
Adhering to local, national, and international regulations to ensure the safety and efficacy of our products.
Utilizing state-of-the-art manufacturing technologies to produce high-quality pharmaceuticals.
Developing treatments for a wide range of conditions, including infectious diseases, oncology, neurology, and cardiovascular diseases.